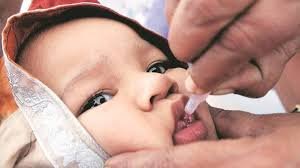
Hyderabad, Feb.12 (RAHNUMA): Hyderabad-based Biological E. Limited has secured Phase II Pre-Qualification (PQ) from the World Health Organization (WHO) for its Novel Oral Polio Vaccine type 2 (nOPV2), enabling full end-to-end manufacturing at a single integrated facility.
The approval covers both drug substance and drug product manufacturing, expanding on the Phase I PQ granted in June 2024. The company has already supplied 700 million doses of nOPV2 to global stockpiles supporting vaccination drives against circulating vaccine-derived poliovirus type 2 (cVDPV2).
The milestone strengthens global outbreak response capacity by adding integrated WHO-qualified production, enhancing supply resilience and rapid deployment during emergencies. Biological E. Managing Director Mahima Datla said faster response and reliable supply are critical to halting transmission during outbreaks.
Mahima further said, “When polio outbreaks occur, response time and vaccine availability determine how quickly transmission can be stopped. nOPV2 has already been deployed in over a billion doses globally because it is designed specifically for outbreak control with improved genetic stability. With Phase II WHO Pre-Qualification enabling full end-to-end manufacturing at our facilities, global supply becomes more resilient and more responsive. That matters because every delay in outbreak response carries real risk for children and communities.”
The achievement follows multi-year global collaboration involving PT Bio Farma (Indonesia), PATH, and the Gates Foundation. With validated processes and export approvals in place, the company aims to support evolving global demand and accelerate polio eradication efforts.